Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column (group 2, or alkaline earth metals) of the periodic table: all group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure.
Read more...
BEFORE YOU WATCH.
Starter: What do you know of the characteristics of magnesium and its chemistry?
1. Watch the video and answer the questions.
A. Complete/answer the following sentences with words or expressions from the video to explain the characteristics of magnesium.
1. COMPLETE: it [magnesium] is the (1) of the (2) metals you can (3) .
2. ANSWER: What is the problem with using lithium and sodium as a metal?
3. ANSWER: What is the problem with using beryllium as a metal?
4. ANSWER: What other advantages is there with choosing magnesium rather than the 3 other elements mentioned?
5. ANSWER: Why was magnesium used for photographic flashlights? What proves the intensity of the reaction?
6. COMPLETE: it's one of the few metals which is meant to burn in (4) . In theory, you can take a piece of magnesium, (5) to it in air so it's burning well and then (6) it into nitrogen gas and it should continue (7) .
7. ANSWER: What is the difference between theory and practice for the professor concerning the experiment?
8. ANSWER: What elements form chlorophyll?
Your teacher will give you a book on the first session.
If you wish to have a digital copy you can download it here or from Madoc.
Online activities available on Madoc are compulsory and must be completed by session 6 at the latest.
Read the text and answer the questions.
Since the birth of synthetic chemistry about 180 years ago, society has loved the wonders bestowed by the field, such as life-saving medicines, pest control and molecules that light up telephone displays, yet has harshly criticized it as being solely responsible for pollution and environmental harm. Synthetic biology emerged as an alternative for constructing molecules only about ten years ago, but some have already proclaimed that it will supplant chemical synthesis. No one should doubt the usefulness of synthetic biology, or its potential to shorten synthetic routes and reduce waste in chemical production. But we are convinced that synthetic chemistry will continue to dominate for the foreseeable future (...), for three main reasons.
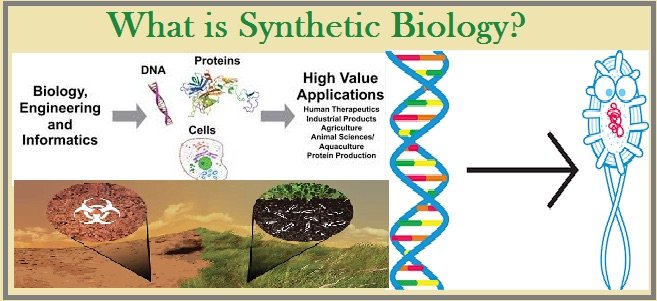
Synthetic biology example.
Credit: jagranjosh.com.
The first reason is that chemical synthesis is the best way to solve supply problems. For decades, synthetic chemistry has provided sufficient quantities of agrochemicals, medicines, perfumes and materials for society’s needs. The pharmaceutical industry in particular relies on chemical methods for the large-scale production of most small-molecule drugs. The majority of these compounds are based on molecular structures not found in nature, which means that they cannot be prepared through enzymatic processes and are likely to be toxic to the host organisms used in synthetic biology. Synthetic biology has had a crucial impact on the commercial production of some medicines derived from complex natural products, such as artemisinin and the anticancer drug paclitaxel (Taxol) But natural products are essentially the only compounds for which biological syntheses can compete with chemical ones, because evolution has optimized the biosynthesis of those products over time.
So the supply of chemicals is best addressed by synthetic chemistry, unless a specific natural product is required in large quantities — and even then, semi-synthetic strategies involving a few chemical steps are often required. In fact, total chemical syntheses of natural products are becoming increasingly efficient and scalable, as demonstrated by the impressive routes used to make tetracycline antibiotics and the anticancer agent eribulin. A practical chemical synthesis of artemisinin has also now emerged that could form the basis of an industrial-scale process for making the drug, and a large-scale synthesis of Taxol is being developed.
Synthetic chemistry example.
Credit: Science magazine.
Optimizing the properties of useful compounds, or adapting their functions to new applications, often requires modification of their molecular structures. The second reason that synthetic chemistry will endure is that chemical methods provide a reliable set of tools to do this in many fundamentally different cases. Moreover, unlike biological syntheses, chemical syntheses can often be developed and implemented rapidly, which is a great advantage.
The third reason is that chemistry excels in the invention of unnatural molecules that have desirable physical properties — such as dyes for printable organic solar cells, fluorescent probes for biological research or radiolabelled drugs used in medicine. The molecular needs of vibrant modern fields such as supramolecular chemistry, chemical biology and nanotechnology can be addressed only by synthetic chemistry.
Source: practical-chemistry.com
Read the text on practical chemistry and say whether the following statements are true or false.
1. According to the authors, synthetic chemistry has more drawbacks than advantages.
2. Synthetic chemistry emerged earlier than synthetic biology.
3. The authors think that synthetic biology will replace synthetic chemistry.
4. Synthetic chemistry is used to prepare compounds that are hard to obtain from natural sources.
5. Compounds used in the pharmaceutical industry are not dangerous for living organisms.
6. The synthesis of a natural compound necessarily involves a biological approach.
7. Synthetic biology is often quicker than chemical synthesis.
8. One advantage of synthetic chemistry is it can quickly and efficiently adapt to innovative technologies.
Choose the best answer to the following questions
1. What advantage of synthetic biology over synthetic chemistry is acknowledged by the authors?
2. Which industry using chemical synthesis is NOT mentioned by the authors?
Fill in the following table with words or expressions from the text that correspond to the definitions
| Line | Synonyms/definitions | Words from the text |
|---|---|---|
| presented | ||
| a destructive insect or other animal that attacks crops, food or livestock | ||
| not involving anyone or anything else | ||
| predictable | ||
| stock of a resource | ||
| a living cell in which a virus multiplies | ||
| measurable | ||
| a device used to carry out a particular function | ||
| put into practical effect | ||
| substance used to add a color to or change the color of something |
2 Rue de la Houssinière
Building 2 - Office 109
Nantes 44322 cedex 3